End of the COVID-19 Public Health Emergency

Fact Sheet: End of the COVID-19 Public Health Emergency Based on current COVID-19 trends, the Department of Health and Human Services (HHS) is planning for the federal Public Health Emergency […]
White House and HRSA COVID-19 Vaccination Webinar

White House and HRSA COVID-19 Vaccination Webinar In light of the recent Expanding COVID-19 Vaccination (ECV) funding, HRSA invites health centers to join a webinar we’re co-hosting with the […]
Long COVID and Fatiguing Illness Recovery Program

Long COVID and Fatiguing Illness Recovery Program Family Health Centers of San Diego, Project ECHO, University of Washington and University of Colorado have collaborated to provide a CDC-funded monthly learning […]
Due Sunday: Expanding COVID-19 Vaccination Funding Application

Due Sunday: Expanding COVID-19 Vaccination Funding Application Submissions Health centers have until Sunday, January 8, to submit information about Expanding COVID-19 Vaccination (ECV) planned activities and costs in HRSA’s Electronic […]
Expanding COVID-19 Vaccination Funding Applications

Expanding COVID-19 Vaccination Funding Applications Health centers have until Sunday, January 8, 2023, to submit information about Expanding COVID-19 Vaccination (ECV) planned activities and costs in HRSA’s Electronic Handbooks (EHBs). […]
Get COVID-19 at-home tests shipped to you at no cost

As COVID-19 cases rise, it’s important to stay safe this winter. Each U.S. household can now order 4 COVID-19 at-home tests shipped straight to their door at no cost. All you […]
HHS Announces New $350 Million Initiative to Increase COVID-19 Vaccinations
HHS Announces New $350 Million Initiative to Increase COVID-19 Vaccinations HHS will distribute funding to health centers to support community-based vaccination events and outreach focused on underserved populations The U.S. […]
Rural Health Clinic COVID-19 Vaccine Distribution (RHCVD) Program

Rural Health Clinic COVID-19 Vaccine Distribution (RHCVD) Program RHCVD Program Summary The Rural Health Clinic Vaccine Distribution Program (RHCVD) distributes COVID-19 vaccines directly to Rural Health Clinics (RHCs) to increase […]
COVID-19: Boosting with an mRNA vaccine offers better protection in people who received two doses of CoronaVac
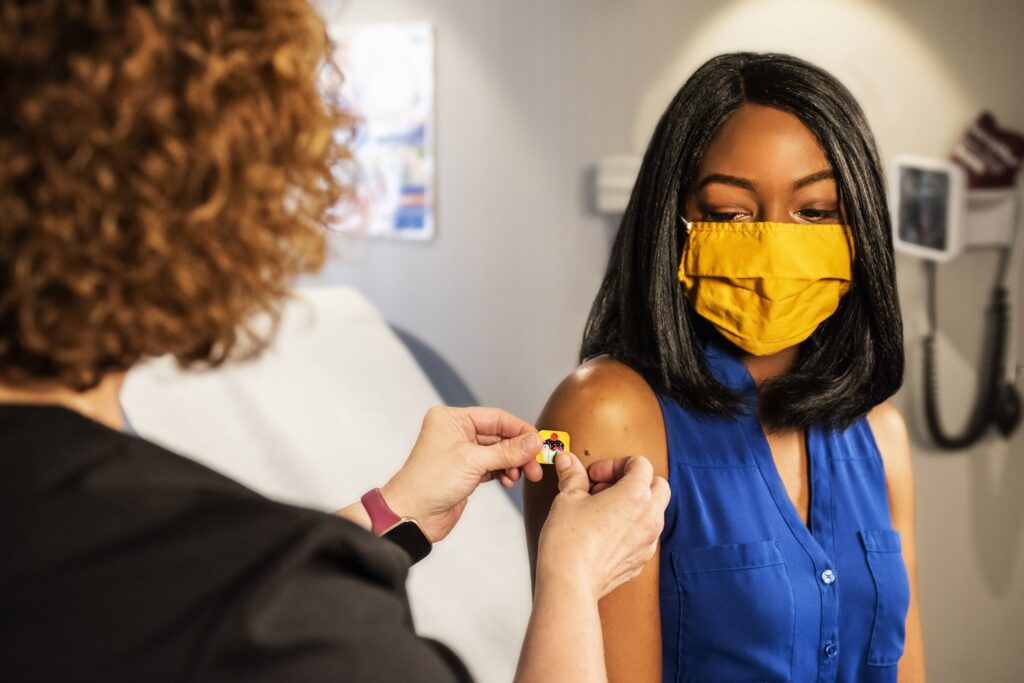
COVID-19: Boosting with an mRNA vaccine offers better protection in people who received two doses of CoronaVac by Barcelona Institute for Global Health One year after mass vaccination against COVID-19 […]
Updated COVID-19 Vaccines Providing Protection Against Omicron Variant Available at No Cost

Updated COVID-19 Vaccines Providing Protection Against Omicron Variant Available at No Cost The Department of Health & Human Services (HHS), through the Centers for Medicare & Medicaid Services (CMS), has […]

